Day :
- Track 10: Tissue Engineering and Regenerative Medicine
Track 14: 3D Printing of Biomaterials
Track 5: Biomaterials Applications

Chair
Elisabeth Engel
University of Catalonia, Spain

Co-Chair
Miguel Angel Mateos Timoneda
CIBER of Bioengineering, Biomaterials and Nanomedicine, Spain
Session Introduction
Elisabeth Engel
University of Catalonia, Spain
Title: Tailoring microenvironments for in situ regeneration
Time : 10:45-11:10

Biography:
Elisabeth Engel is an Associate Professor at Technical University of Catalonia since 2010. She received her PhD in 2003 in Bone Metabolism Diseases from a Medical School. She was appointed as PI at the Group of Biomaterials for Regenerative Therapies since September, 2012 at the Institute for Bioengineering of Catalonia. Her research interests include the preparation and design of materials and scaffolds for in vitro and in vivo fundamental studies, and a further focus is the provision of useful tools to assess mechanisms that govern cell behaviour in regenerative medicine.
Abstract:
At present, tissue engineering for bone regeneration seeks to obtain scaffolds that mimic the cell microenvironment to recruit stem and progenitor cells to recapitulate the development of target tissues. Herein, we have explored the use of citric acid related to bone nanostructure and mechanical performance, to develop scaffolds resembling the extracellular matrix of developing bone. Elastin-like recombinamers (ELRs) hydrogels were achieved through a one-step chemical crosslinking reaction with citric acid, a molecule currently considered to be essential for the proper performance of bone tissue. We were able to control the architecture and stiffness of citric acid-crosslinked hydrogels while preserving the integrity of adhesion sequences in ELRs. Interestingly, the use of citric acid conferred so-produced hydrogels the ability to nucleate calcium phosphate. In vivo implantation of both mechanically-tailored and non-tailored citric acid-crosslinked hydrogels demonstrated to be able to mineralize the new formed tissue and to integrate into bone in critical size defects in mouse calvaria. Both types of hydrogels showed bone tissue formation by intramembranous ossification. The non-mechanically tailored scaffold showed higher cellular activity (in terms of osteoblasts and osteoclasts presence) related to a lower density of the matrices that allowed higher cell penetration.

Recent Publications:
1. Sánchez Ferrero A, Mata Á, Mateos Timoneda MA, Rodríguez Cabello JC, Alonso M, Planell J, Engel E (2015) Development of tailored and self-mineralizing citric acid crosslinked hydrogels for in situ bone regeneration. Biomaterials 68:42-53
2. Mendes AC, Smith KH, Tejeda-Montes E, Engel, E, Reis RL, Azevedo HS, Mata A (2013) Co-assembled and microfabricated bioactive membranes. Advanced Functional Materials 23(4): 430-438
3. Levato R, Planell JA, Mateos-Timoneda MA, Engel E (2015) Role of ECM/peptide coatings on SDF-1α triggered mesenchymal stromal cell migration from microcarriers for cell therapy. Acta Biomater. 18:59-67
4. Oliveira Hugo, Catros Sylvain, Boiziau Claudine, Siadous Robin, Martiâ€Munoz Joan, Bareille Reine, Rey Sylvie, Castano Oscar, Planell Josep, Amédée Joëlle, Engel Elisabeth (2016) The proangiogenic potential of a novel calcium releasing biomaterial: Impact on cell recruitment Acta Biomaterialia 29:435â€445
5. Álvarez Z, Castaño O, Castells AA, Mateos-Timoneda MA, Planell JA, Engel E, Alcántara S (2014) Neurogenesis and vascularization of the damaged brain using a lactate-releasing biomimetic scaffold. Biomaterials 3517:4769-4781.
Avelina Sotres Vega
National Institute of Respiratory Diseases, Mexico
Title: Biomaterials as a tool for teaching and learning training programs on surgery
Time : 11:10-11:35

Biography:
Avelina Sotres Vega has her expertise in teaching and learning programs on surgery using preserved biomaterials either by cryopreservation or lyophilization as well as cryopreserved tracheal grafts in experimental models of long segment replacement mainly.
Abstract:
Statement of the Problem: Developing surgical skills is essential in the training of all surgical specialties. However ethical, legal and economic issues have limited surgical training. As surgical educators, we are always trying to identify new ways to provide skills training. We have developed training programs to teach surgical skills to junior surgeons from human and veterinary medicine based on laboratory animals using preserved tissues and organs as surgical training biomaterials. The goal is to help the trainees acquire the abilities and dexterity necessary to perform surgery on patients.
Methodology: We created a small bank of cryopreserved tracheas and stomachs harvested from Wistar rats as well as cryopreserved tracheas and lyophilized esophagus that were harvested from dogs. All animals were previously used in research studies. Stomachs, tracheas and esophagus were cleansed with saline solution, after this, tracheas and esophagus were mounted on polypropylene tubes. In the stomachs, the pyloric antrum was tied with silk 1-0, stomachs were filled with hyaluronic acid solution and the distal esophagus was tied too. Tracheas and esophagus were trimmed in segments of 5 cm. Cryopreservation of tracheas and stomachs was made with a controlled-rate freezer (-1ºC/min) and stored at -30ºC for 30 to 60 days. All the esophagus were lyophilized at -55ºC and 10 mBar of vacuum pressure during 24 hours and sterilized with low temperature hydrogen peroxide gas plasma process. On the day of the surgical skills practice, the cryopreserved organs were thawed at room temperature and all the esophagus were rehydrated with saline solution at 4ºC. Each preserved organ was used to perform end-to-end anastomosis with 4-0 running polypropilene or single stitches. Preserved organs are inexpensive, practical, portable bench models and have high-fidelity that improve the tactile perception and facilitate surgical skills learning by improving how trainees handle tissue and surgical instruments.
Recent Publications:
1. Sotres-Vega A, Villalba-Caloca J, Azrad-Daniel S, García-Montes JA, Torre-Jaime JL, Guadarrama-Sánchez I, Pérez-Covarrubias D, Santibañez-Salgado JA (2016). Surgical Skills Training with Cryopreserved Rat Stomachs. Journal Veterinary Medical Education 43(4):420-426.
2. Giraldo-Gomez DM, Leon-Mancilla B, Del Prado-Audelo ML, Sotres-Vega A, Villalba-Caloca J, Garciadiego-Cazares D, Piña-Barba MC (2016). Trypsin as enhancement in cyclical tracheal decellularization: Morphological and biophysical characterization. Materials Science & Engineering. C, Materials for Biological Applications (2016) 59:930-7. doi: 10.1016/j.msec.2015.10.094.
3. J. Pineda-Gutiérrez, A. Sotres-Vega, J. Villalba-Caloca, M. Alonso-Gómez, S. Aja-Guardiola, J. García-Jarquín, S. Martínez-Fonseca, J. Salas-Hernández (2016). Preservation of cardiopulmonary blocks: A real and interactive biomaterial for teaching and learning lung plethysmography and mechanical ventilation. International Journal of Engineering Research & Science (IJOER) 2(4):162-166.
4. Olmos-Zúñiga JR, Jasso-Victoria R, Díaz-Martínez NE, Gaxiola-Gaxiola MO, Sotres-Vega A, Heras-Romero Y, Baltazares-Lipp M, Baltazares-Lipp ME, Santillán-Doherty P, Hernández-Jiménez C (2016). Lyophilized allografts without pre-treatment with glutaraldehyde are more suitable than cryopreserved allografts for pulmonary artery reconstruction. The Brazilian of Medical and Biological Research 49(2):e5001. doi: 10.1590/1414-431X20155001.
5. Sotres-Vega A, Osorio-Necoechea ME, Salas-Galindo G, González-Ramón SC, Guadarrama-Sánchez I, Villalba-Caloca J, Santibáñez-Salgado JA (2013). Bench surgical training with lyophilized esophageal segments. Acta Cirurgica Brasileira 28(8):619-23.
Miguel Angel Mateos Timoneda
CIBER of Bioengineering, Biomaterials and Nanomedicine, Spain
Title: Design of a Composite Bioink for Bioprinting Applications
Time : 11:35-12:00

Biography:
M A Mateos-Timoneda is an expert in the field of Biomaterials and Scaffolds for Tissue Engineering. He holds a PhD in Supramolecular Chemistry from the University of Twente (Enschede, The Netherlands). Since 2007, he is a Senior Researcher at CIBER en Bioingeniería, Biomateriales y Nanomedicina (CIBER-BBN) and in the Biomaterials for Regenerative Therapies Group at the Institute for Bioengineering of Catalonia (IBEC) in Barcelona (Spain). His main interests are the study of cell delivery using biodegradable microcarriers and 3D printing and bioprinting.
Abstract:
3D bioprinting is an expanding field that allows the fabrication of customized tissue engineered scaffolds with encapsulated cells. Designing a biomaterial fit for 3D printing and cell encapsulation (bioink) is a complex task due to the high number of requirements that need to be accomplished. Bioinks are usually based on combinations of different hydrogels due to their encapsulation capacities, but other kinds of materials can be added in order to improve certain characteristics of the final scaffold. A novel composite bioink that includes alginate as a printable hydrogel and calcium-releasing particles as a vascularization promoter is optimized and studied. Rheometry studies show that the addition of calcium-releasing particles to alginate increases its viscosity, but does not alter its shear thinning properties; therefore maintaining the printability of the material. Solid scaffolds with theoretically high nutrient diffusion rates (filament diameter ≈ 200 µm) are printed using this novel bioink and a novel cross-linking method. A bioprinting procedure that includes encapsulation of cells is optimized and tried out successfully with different bioinks, obtaining good survival rates. The addition of calcium releasing-particles improves cell survival after the bioprinting process as well as during the first culture days. Moreover, the interaction between calcium-releasing particles and alginate is proven to be adequate for bioprinting and could be an interesting line of research for bone regeneration and tissue vascularization applications.
Recent Publications:
1. Levato R, Visser J, Planell JA, Engel E, Malda J, Mateosâ€Timoneda MA (2014) Biofabrication of tissue constructs by 3D bioprinting of cellâ€laden microcarriers. Biofabrication 6:035020.
2. Puñet X, Machauffé R, Giannotti MI, Rodríguezâ€Cabello JC, Sanz F, Engel E, Mateosâ€Timoneda MA, Planell JA (2013) Enhanced cell†material interactions through the biofunctionalization of polymeric surfaces with engineered peptides. Biomacromolecules 14:2690.
3. Levato R, Planell JA, Mateosâ€Timoneda MA, Engel E (2015) Role of ECM/peptide coatings on SDFâ€1a triggered mesenchymal stromal cell migration from microcarriers for cell therapy. Acta Biomater 18:59.
4. Baelo A, Levato R, Julián E, Crespo A, Astola J, Gavaldà J, Engel E, Mateosâ€Timoneda MA, Torrents E (2015) Disassembling bacterial extracellular matrix with DNaseâ€coated nanoparticles to enhance antibiotic delivery in biofilm infections. J Control Release 209:150.
5. Levato R, Mateosâ€Timoneda MA, Planell JA (2012) Preparation of biodegradable polylactide microparticles via a biocompatible procedure. Macromol Biosci 12:557.
Sybille Facca
University of Strasbourg , France
Title: Development of nanostructured biomaterials for bone and osteo-articular regeneration
Time : 12:00-12:25

Biography:
Sybille Facca, MD, PhD, has her expertise in Orthopedic, Hand and Nerve Surgery as an Orthopedic Surgeon at Strasbourg Hospital University since 2007. She was the first person focusing her research on bone and cartilage regeneration and drug delivery systems of bone cements or nanofibers membranes and osteointegration of orthopaedic implants. Now, she is also focusing her research on tubes for nerve regeneration, microsurgery simulation or microanastomosis mechanical properties and new design of wrist arthroplasty, in a biomechanical laboratory of Strasbourg University.
Abstract:
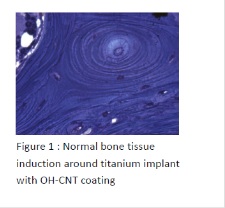
During the last ten years, tissue engineering has merged with regenerative nanomedicine by combination, not only of new biomaterials but also of stem cell technology and growth factors. The goal of this work was to use bone and cartilage engineering as a model, in order to improve and to develop active and living nanostructured implants. We were interested in the development of biomaterials (natural or synthetic), tridimensional (3D), transplantable for bone and cartilage diseases treatments, that are able to induce more cellular differentiation and improved tissue regeneration. We have developed 3 types of nanostructured implants, (i) titanium implants coated with hydroxyapatite and carbon nanotubes in order to improve osteoformation and osteoinduction around arthroplasty implants; (ii) active capsules functionalized by growth factors and stems cells for bone induction (in vitro/in vivo) after a bone defect; (iii) electrospun nanofibrous membranes functionalized by growth factors and (Osteoblasts/Chondrocytes) for bone and cartilage regeneration, in vitro and in vivo.
Recent Publications:
1. Facca S, Lahiri D, Fioretti F, Messadeq N, Mainard D, Benkirane-Jessel N, Agarwal A (2011) In vivo osseointegration of nano-designed composite coatings on titanium implants. ACS Nano 5:4790-4799.
2. Facca S, Lahiri D (2012) Nanoreinforcement of hydroxyapatite coatings on titanium for osseointegration of orthopaedic implants. Comput Methods Biomech Biomed Engin. 15 Suppl 1:10-11.
3. Eap S, Keller L, Ferrand A, Schiavi J, Lahiri D, Lemoine S, Facca S, Fioretti F, Mainard D, Agrawal A, Benkirane-Jessel N (2014) Nanomechanical properties of active nanofibrous implants after in vivo bone regeneration. Nano Life 04(01) DOI: 10.1142/S1793984414500019.
4. Vaiss L, Ichihara S, Ramirez DG, Hendriks S, Liverneaux P, Facca S (2015) A comparative study about ionizing radiation emitted during radiological "skyline" view of the wrist in pronation versus supination. Eur J Orthop Surg Traumatol 25:309-311.
5. Perruisseau-Carrier A, Bahlouli N, Po C, P Vernet, Facca S , Liverneaux P (2017) Analysis of the modifications of MRI signal of the brachial plexus of rats: Comparative study before and after freezing/thawing. Ann Chir Plast Esthet pii: S0294-1260 (16) 30214-X.
Jose Manuel Baena
BRECA Health Care, Spain
Title: Design and characterization of bioinks with hyaluronic acid for tissue and bone-3D bioprinting
Time : 12:25-12:50

Biography:
José Manuel Baena, MSc is a Research Associate. He is the Founder of BRECA Health Care, pioneer in 3D printed custom made implants for orthopedic surgery, and REGEMAT 3D, the first Spanish bioprinting company. He is an expert in innovation, business development and internationalization. He is a Lecturer in some business schools, and is passionate about Biomedicine and Technology. In his free time, he also works as a Researcher at the Biopathology and Regenerative Medicine Institute (IBIMER).
Abstract:
Statement of the Problem: The 3D bioprinting of tissues and organs represents a major breakthrough in regenerative medicine and tissue engineering. Cartilage and bone regeneration provides an alternative in the treatment of diseases such as degenerative osteoarthritis, injuries of articular cartilage, osteonecrosis and bone fractures, among others. The purpose of this study is to describe the design, development and preparation of a bioink with hyaluronic acid (HA) to manufacture cartilage and bone by 3D bioprinting.
Methodology & Theoretical Orientation: For the formulation of bioinks, two hyaluronic acids were studied: high molecular weight sodium hyaluronate (bioinkA) and low molecular weight sodium hyaluronate (bioinkB), both of intra-articular grade. The HA was combined with alginate and human chondrocytes. The biopaper studied was the polylactic acid (PLA). Cell viability was studied for each bioink.
Findings: The results obtained showed that the HA concentration before and after the bioprinting process did not affect chondrocyte viability. Additionally, cells remained in proliferation after 5 weeks. The rheological properties of each bioink showed mild differences between bioinkA and bioinkB.
Conclusion & Significance: Considering the mild differences in rheological properties between the two experimental bioinks, it may be concluded that both formulations can be used for cartilage and bone bioprinting.
Recent Publications:
1. Gálvez-Martín P, Hmadcha A, Soria B, Calpena-Campmany AC, Clares-Naveros B (2014) Study of the stability of packaging and storage conditions of human mesenchymal stem cell for intra-arterial clinical application in patient with critical limb ischemia. European Journal of Pharmaceutics and Biopharmaceutics 86:459-468.
2. Gálvez P, Clares B, Bermejo M, Hmadcha A, Soria B (2014) Standard requirement of a microbiological quality control program for the manufacture of human mesenchymal stem cells for clinical use. Stem Cells and Development 23:1074-1083.
3. Gálvez P, Martín MJ, Calpena AC, Tamayo JA, Ruiz MA, Clares B (2014) Enhancing effect of glucose microspheres in the viability of human mesenchymal stem cell suspensions for clinical administration. Pharmaceutical Research 31:3515-3528.
4. Martín MJ, Calpena AC, Fernández F, Mallandrich M, Gálvez P, Clares B (2015) Development of alginate microspheres as nystatin carriers for oral mucosa drug delivery. Carbohydrate Polymers 117:140-149.
5. Llavero F, Urzelai B, Osinalde N, Gálvez P, Lacerda HM, Parada LA, Zugaza JL (2015) Guanine nucleotide exchange factor αPIX leads to activation of the Rac 1GTPase/Glycogen phosphorylase pathway in interleukin (IL)-2-stimulated T cells. Journal of Biological Chemistry 290:9171-9182.
Deniz Yucel
Acıbadem University, Turkey
Title: The effect of scaffold topography on behavior of dental pulp stem cells
Time : 13:50-14:15

Biography:
Abstract:
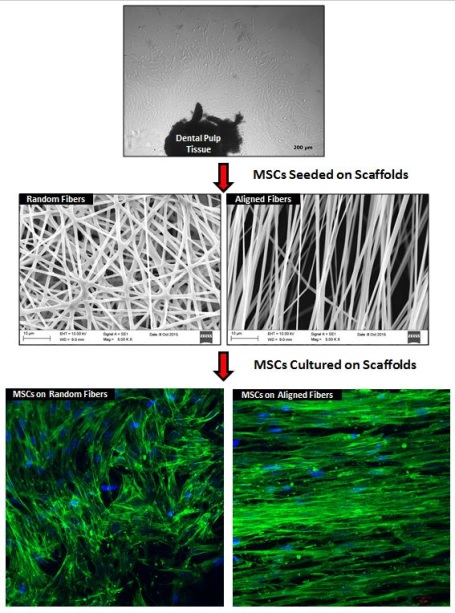
Cristina Plamadeala
Johannes Kepler University Linz, Austria
Title: Bio-inspired microstructures for directional liquid transport
Time : 14:15-14:40

Biography:
Abstract:

- Track 1: Advanced Biomaterials
Track 7: Biomaterials and Nanotechnology

Chair
Monica Lopez Fanarraga
UNIVERSITY OF CANTABRIA-IDIVAL, Spain

Co-Chair
Isabel Rodriguez
IMDEA-Nanoscience, Spain
Session Introduction
Mónica López Fanarraga
UNIVERSITY OF CANTABRIA-IDIVAL, Spain
Title: Bio-degradable carbon nanotubes display intrinsic anti-tumoral effects
Time : 14:40-15:05

Biography:
Abstract:
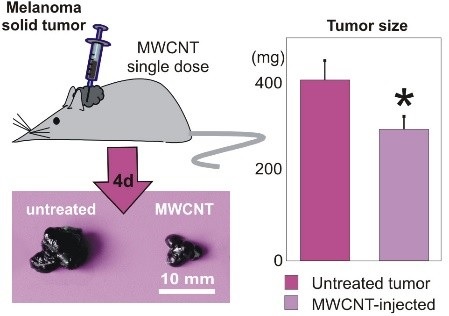
1. Garcíaâ€Hevia L et al. (2015) Multiwalled carbon nanotubes interfere with cell biomechanics inhibiting cancer cell migration. Adv. Healthc. Mat. 4:1640â€4.
2. Villegas J et al. (2014) Multiwalled carbon nanotubes hinder brain macrophage function interfering with cell migration and phagocytosis. Adv. Healthc. Mat. 3:424-32.
3. García-Hevia L et al. (2015) Antiï€cancer cytotoxic effects of multiwalled carbon nanotubes. Curr. Pharm. Design. 21:1920-1929.
4. García-Hevia L et al. (2016) Multiwalled carbon nanotubes inhibit tumor progression in a mouse model. Adv. Healthc. Mat. 5:1080-7.
5. Garcíaâ€Hevia L et al. (2016) Nanoâ€ZnO leads tubulin macrotube assembly and actin bundling triggering cytoskeletal catastrophe and cell necrosis. Nanoscale. 8:10963â€73.
Isabel Rodriguez
IMDEA-Nanoscience, Spain
Title: Modulation of cell behavior on artificial materials by functional nano-topographies for applications in regenerative medicine
Time : 15:05-15:30

Biography:
Abstract:

2. Ho AY, Luong-Van E, Lim CT, Natarajan S, Elmouelhi N, Low H Y, Vyakarna M, Cooper K and Rodriguez I (2014) Lotus bioinspired superhydrophobic, self-cleaning surfaces from hierarchically assembled templates. Journal of Polymer Science Part B: Polymer Physics 52: 603-609.
3. Rodriguez I, Lim C, Natarajan S, Ho YY, Luong van E, Elmouelhi N , Low HY, Vyakarnam M and Cooper K (2013) Shear adhesion strength of gecko-inspired tapes on surfaces with variable roughness. Journal of Adhesion. 89: 921-936.
4. Luong-Van E, Rodriguez I, Low HY, Elmouelhi N, Lowenhaupt B, Natarajan S, Lim CT, Prajapati R, Vyakarnam M and Cooper K (2013) Micro- and nanostructured surface engineering for biomedical applications. Journal of Materials Research 28: 165-174.
5. Lin L, Chu YS, Thiery JP, Lim CT, Rodriguez I (2013) Microfluidic cell trap array for controlled positioning of single cells on adhesive micropatterns. Lab on Chip 13: 714-721.
Anna Cifuentes-Rius
Monash University, Australia
Title: Two stage, dual action cancer therapy with targeted porous silicon nanoparticles
Time : 15:30-15:55

Biography:
Abstract:
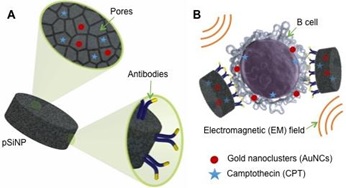
Recent Publications:
1. H Alhmoud, B Delalat, R Elnathan, A Cifuentes-Rius, A Chaix, ML Rogers, JO Durand and NH Voelcker (2015) Title of the abstract. Advanced Functional Materials 25:1137-1145.
Michaela Fousova
UCT Prague, Czech Republic
Title: Metallic 3D-printing for orthopedic surgery: question of surface and cell compatibility
Time : 16:10-16:35

Biography:
Abstract:
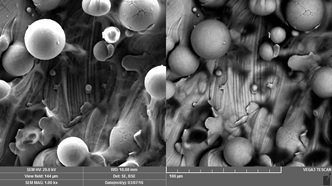
1. Fousová M et al. (2017) Promising characteristics of gradient porosity Ti-6Al-4V alloy prepared by SLM process. Journal of the Mechanical Behavior of Biomedical Materials. In press.
Peng-Sheng Wei
National Sun Yat-Sen University, Taiwan
Title: Stages for Pore Formation during fabrication of Porous materials
Time : 16:35-17:00

Biography:
Abstract:
Recent Publications:
1. Wei PS, Chao TC (2016) The effects of drilling parameters on pore size in keyhole mode welding. ASME Journal of Manufacturing Science and Engineering; 138: 021008.
2. Wei PS, Chang CC (2016) Existence of universal phase diagrams for describing general pore shape resulting from an entrapped bubble during solidification. ASME Journal of Heat Transfer; 138: 104503.
3. Wei PS, Hsiao SY (2016) Effects of mass transfer coefficient on pore shape in solid. International Journal of Heat and Mass Transfer; 103: 931-939.
4. Wei PS, Hsiao SY (2016) Effects of solute concentration in liquid on pore shape in solid. International Journal of Heat and Mass Transfer; 103: 920-930.
- Track 2: Polymer Biomaterials
Track 3: Dental Biomaterials
Track 4: Properties of Biomaterials

Chair
Delair Thierry
University of Lyon, France

Co-Chair
Jose Ramon Sarasua
University of the Basque Country, Spain
Session Introduction
Thierry Delair
University of Lyon, France
Title: Polyelectrolyte nano-complexes-Safe and Efficient tools for the delivery of drugs or vaccine
Time : 10:35-11:00

Biography:
Abstract:
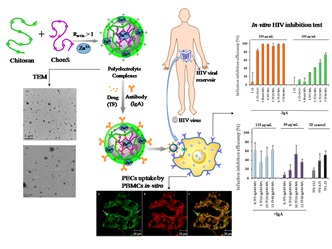
Recent Publications:
1. Wu, D., Delair, T. (2015) Stabilization of chitosan/hyaluronan colloidal polyelectrolyte complexes in physiological conditions, Carbohydrate Polymers 119, 149-158.
2. Costalat, M., Alcouffe, P., David, L., and Delair, T. (2015) Macro-hydrogels versus nanoparticles by the controlled assembly of polysaccharides, Carbohydrate Polymers 134, 541-546.
3. Costalat, M., David, L., and Delair, T. (2014) Reversible controlled assembly of chitosan and dextran sulfate: A new method for nanoparticle elaboration, Carbohydrate Polymers 102, 717-726.
4. Polexe, R. C., Terrat, C., Verrier, B., Cuvillier, A., Champier, G., and Delair, T. (2013) Elaboration of targeted nanodelivery systems based on colloidal polyelectrolyte complexes (PEC) of chitosan (CH)-dextran sulphate (DS), European Journal of Nanomedicine 5.
5. Delair, T. (2011) Colloidal polyelectrolyte complexes of chitosan and dextran sulfate towards versatile nanocarriers of bioactive molecules, European Journal of Pharmaceutics and Biopharmaceutics 78, 10-18.
Jose Ramon Sarasua
University of the Basque Country, Spain
Title: Biodegradable polyesters for biomedical applications: alternatives to polylactides and polylactones
Time : 11:15-11:40

Biography:
Abstract:
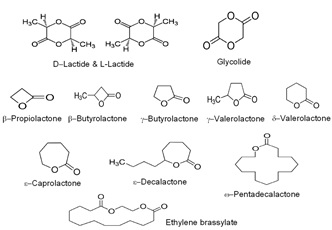
Recent Publications:
1. Jose R Sarasua, Etxeberria A and Fernández J (2016) Synthesis and properties of ω-​pentadecalactone-​co-​δ-hexalactone copolymers: A biodegradable thermoplastic elastomer as an alternative to poly (ε-​caprolactone). Rsc Advances 6: 3137-3149.
2. Jose R Sarasua, Fernández J, Amestoy H, Larrañaga-Varga A, Sardon H and Aguirre M (2016) Effect of molecular weight on the physical properties of poly (ethylene brassylate) homopolymers J Mech Behav Biomed Mater 64: 209-219.
3. Jose R Sarasua, Fernández J, Larrañaga A and Etxeberria A (2016) Ethylene brassylate-co-δ-hexalactone biobased polymers for application in the medical field: Synthesis, characterization and cell culture studies. Rsc Advances 6: 22121-22136.
4. Jose R Sarasua, Fernández J, Larrañaga A and Etxeberria A (2014) Tensile behavior and dynamic mechanical analysis of novel poly (lactide/d-valerolactone) statitical copolymers. J. Mech. Behav. Biomed Mater 35: 39-50.
5. Jose R Sarasua and Larrañaga A (2016) Poly (α-hydroxy Acids)-based cell microcarriers. Applied Sciences 6(436): 1-16.
Ruchi Singla
Chandigarh Engineering College, India
Title: A Novel design of low cost Mastitis level measurement based on electrical resistivity
Time : 11:40-12:05

Biography:
Abstract:
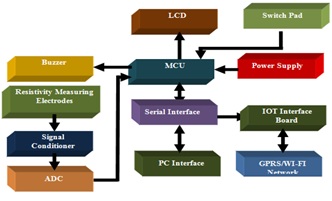
Saurabh Gupta
Axiss Dental Pvt Ltd, India
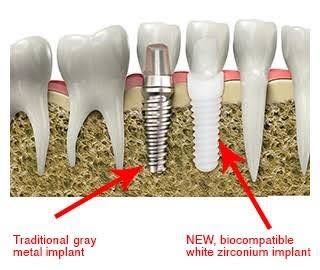
Title: An analysis of dental implant materials with an exclusive focus on Zirconia Vs Titanium
Time : 12:05-12:30

Biography:
Abstract:
Recent Publications:
1. S Gupta (2016) A recent updates on zirconia implants: A literature review. Dent Implants Dentures 1:1.
2. Gupta S (2016) Zirconia vs. titanium implants – Deciding factors. J Dent Oral Disord Ther 4(4): 1-2.
3. Saurabh G (2016) Implant stability measure: Critical review of various methods. Dent Implants Dentures 1:110.
4. Gupta S (2015) A review of perimplantitis and lasers. IOSR Journal of Dental and Medical Sciences 14(3) Ver. I:01-04.
5. Gupta S (2016) Laser-assisted cosmetic dentistry. J Dent Lasers 10:16-8.
Nour el Houda
University of Monastir, Tunisia
Title: The use of Silica nano-particles and microwave irradiation in dental PMMA repairs: Experimental investigation into the mechanical properties and dimensional stability of the repaired PMMA after aging
Time : 12:30-12:55

Biography:
Abstract:

Poster Presentations:14:00-15:00@Avila
- Poster Presentations
Session Introduction
Jose Manuel Baena
BRECA Health Care, Spain
Title: Development of scaffolds for Regenerative Medicine

Biography:
Abstract:
Marco Consumi
University of Siena, Italy
Title: Novel extended-released polymeric matrix for Monascus fermented rice extract (RYR) delivery

Biography:
Abstract:
Richard M Hall
University of Leeds, UK
Title: Wear particle embedded 3D agarose gels for biocompatibility testing of orthopedic medical devices

Biography:
Abstract:
Na Keum Jang
Raphas Co. Ltd., South Korea
Title: Dissolving microneedles: An attractive approach to transdermal drug delivery

Biography:
Abstract:
Patricia Mazon Canales
Universidad Miguel Hernández, Spain
Title: Novel resorbable and osteoconductive nurse’A phase-silicocarnotite scaffold induced bone formation

Biography:
Abstract:
Anna Arvidsson
Dentsply IH, Sweden
Title: Evaluation of anti-biofilm properties of titanium nitride coatings: An in vitro study

Biography:
Abstract:
Richard M Hall
University of Leeds, UK
Title: Isolation of low volumes of silicon nitride particles from tissue

Biography:
Abstract:
Jung Dong Kim
Raphas Co. Ltd., South Korea
Title: Dissolving microneedles: An attractive approach to transdermal drug delivery

Biography:
Abstract:
Eloisa Gonzalez-Lavado
University of Cantabria, Spain
Title: Multi-walled carbon nanotubes (MWCNTs) as cytotoxic drug delivery systems in the treatment of cancer

Biography:
Abstract:
Iordana Neamtu
Petru Poni Institute of Macromolecular Chemistry, Romania
Title: Nanogels preparation for controlled bioactives’ delivery

Biography:
Abstract:
Tiago Monteiro
Universidade Nova de Lisboa, Portugal
Title: Co-immobilization of glucose oxidase and catalase on electrodes’ surface: A multifunctional tool for biosensing applications

Biography:
Abstract:
Aurica P Chiriac
Petru Poni Institute of Macromolecular Chemistry, Romania
Title: New method for magnetic composite deposition onto a stent surface

Biography:
Abstract:
Ingela Mattisson
Dentsply IH, Sweden
Title: Evaluation of anti-biofilm properties of titanium nitride coatings: An in vitro study

Biography:
Abstract:
Zeynep Orman
Yildiz Technical University, Turkey
Title: Production and characterization of bioceramic nanopowders of biological origin

Biography:
Abstract:
Loredana E Nita
Petru Poni Institute of Macromolecular Chemistry, Romania
Title: Nanostructures based on poly(aspartic acid) and bovine albumin by self-assembling procedure

Biography:
Abstract:

Biography:
Abstract:
Award & Closing Ceremony: 16:15 onwards@Avila
