
José Manuel Baena
BRECA Health Care, Spain
Title: An improved biofabrication process to enhance cell survival and distribution in bioprinted scaffolds for cartilage regeneration
Biography
Biography: José Manuel Baena
Abstract
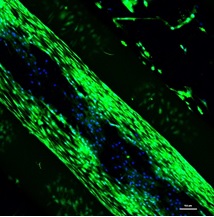
Tissue regeneration (TR) is currently one of the most challenging biotechnology unsolved problems. Tissue engineering (TE) is a multidisciplinary science that aims at solving the problems of TR. TE could solve pathologies and improve the quality of life of billions of people around the world suffering from tissue damages. New advances in stem cell (SC) research for the regeneration of tissue injuries have opened a new promising research field. However, research carried out nowadays with two-dimensional (2D) cell cultures do not provide the expected results, as 2D cultures do not mimic the 3D structure of a living tissue. Some of the commonly used polymers for cartilage regeneration are Poly-lactic acid (PLA) and its derivates as Poly-L-lactic acid (PLLA), Poly(glycolic acids) (PGAs) and derivates as Poly(lactic-co-glycolic acids) (PLGAs) and Poly caprolactone (PCL). All these materials can be printed using fused deposition modelling (FDM), a process in which a heated nozzle melts a thermoplastic filament and deposits it in a surface, drawing the outline and the internal filling of every layer. All these procedures uses melting temperatures that decrease viability and cell survival. Research groups around the world are focusing their efforts in finding low temperature printing thermoplastics or restricted geometries that avoid the contact of the thermoplastic and cells at a higher temperature than the physiologically viable. This mainly has 2 problems; new biomaterials need a long procedure of clearance before they can be used in clinical applications, and restrictions in geometries will limit the clinical application of 3D printing in TE. We have developed an enhanced printing process named Injection Volume Filling (IVF) to increase the viability and survival of the cells when working with high temperature thermoplastics without the limitation of the geometry. We have demonstrated the viability of the printing process using chondrocytes for cartilage regeneration. This development will accelerate the clinical uptake of the technology and overcome the current limitation when using thermoplastics as scaffolds.
Recent Publications:
1. Ti 3D printing and 3D cell bioprinting. New horizons in implantology and maxillofacial surgery. Spanish society of oral and maxillofacial surgery (SECOM) June 2016.
2. 2014 REGEMAT 3D: A Modular Multicomponent Work Station for 3D bioprinting in Regenerative medicine. Current trends and future directions in orthopaedic surgery . Building organs: Milestones and Challenges in tissue engineering Friday, 26th September, 2014.
3.“REGEMAT 3D bioprinting for cartilage regeneration and much more” www.3dbioprintingconference.com January 2016.
4. Real-time probabilistic model-based monitoring bioreactor for engineered cartilage, ESUCB 2013.
5. Proyecto FABIO. Desarrollo de una nueva generación de productos sanitarios personalizados. Ferrís-Oñate J, Morales-Martín I, Blanco-Fernández M, Baena-Martínez JM, Delgado-Gordillo J, Hurtós-Casals E, Atorrasagasti-Goyalde G, Bermejo-Bosch I, Atienza-Vicente CM, Solera-Navarro MJ. Proyecto FABIO. Desarrollo de una nueva generación de productos sanitarios personalizados. Revista de Biomecánica 2010, 53, 75-78.

